

 based on reviews by 2 anonymous reviewers
based on reviews by 2 anonymous reviewers
Mosquitoes are first vector of pathogen worldwide and transmit several arbovirus, most of them leading to major outbreaks (1). Chikungunya virus (CHIKV) is a perfect example of the “explosive type” of arbovirus, as observed in La Réunion Island in 2005-2006 (2-6) and also in the outbreak of 2007 in Italy (7), both vectorized by Ae. albopictus. Being able to better understand CHIKV intra-vector dynamics is still of major interest since not all chikungunya strain are explosive ones (8).
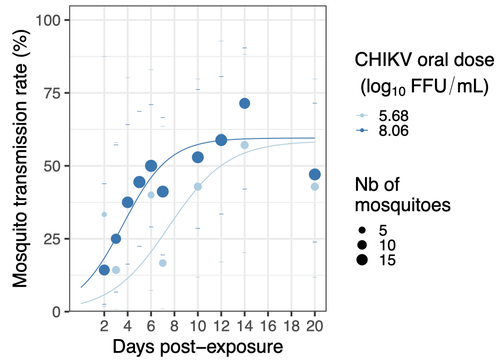
In this study (9), the authors have evaluated the vector competence of a local strain of Aedes albopictus (collected in Lyon, France) for CHIKV. They evaluated infection, dissemination and transmission dynamics of CHIKV using different dose of virus in individual mosquitoes from day 2 to day 20 post exposure, by titration and quantification of CHIKV RNA load in the saliva. As highlighted by both reviewers, the most innovative idea in this study was the use of three different oral doses trying to span human viraemia detected in two published studies (10-11), doses that were estimated through their model of human CHIKV viremia in the blood. They have found that CHIKV dissemination from the Ae. albopictus midgut depends on the interaction between time post-exposure and virus dose (already highlighted by other international publications). Then their results were implemented in the agent-based model nosoi to estimate the epidemic potential of CHIKV in a French population of Ae. albopictus, using realistic vectorial capacity parameters.
To conclude, the authors have discussed the importance of other parameters that could influence vector competence as mosquito microbiota and temperature, parameters that need also to be estimated in local mosquito population to improve the risk assessment through modelling.
As pointed out by both reviewers, this is a nice study, well written and easy to read. These results allow filling in another gap of our understanding of CHIKV intra-vector dynamics and highlight the epidemic potential of CHIKV upon transmission by Aedes albopictus in mainland France. For all these reasons, I chose to recommend this article for Peer Community In Infections.
References
1. Marine Viglietta, Rachel Bellone, Adrien Albert Blisnick, Anna-Bella Failloux. (2021). Vector Specificity of Arbovirus Transmission. Front Microbiol Dec 9;12:773211. https://doi.org/10.3389/fmicb.2021.773211
2. Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney M-C, Lavenir R, Pardigon N, Reynes J-M, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel M-P, Bréhin A-C, Cubito N, Desprès P, Kunst F, Rey FA, Zeller H, Brisse S. (2006). Genome Microevolution of Chikungunya viruses Causing the Indian Ocean Outbreak. 2006. PLoS Medicine, 3, e263. https://doi.org/10.1371/journal.pmed.0030263
3. Bonilauri P, Bellini R, Calzolari M, Angelini R, Venturi L, Fallacara F, Cordioli P, 687 Angelini P, Venturelli C, Merialdi G, Dottori M. (2008). Chikungunya Virus in Aedes albopictus, Italy. Emerging Infectious 689 Diseases, 14, 852–854. https://doi.org/10.3201/eid1405.071144
4. Pagès F, Peyrefitte CN, Mve MT, Jarjaval F, Brisse S, Iteman I, Gravier P, Tolou H, Nkoghe D, Grandadam M. (2009). Aedes albopictus Mosquito: The Main Vector of the 2007 Chikungunya Outbreak in Gabon. PLoS ONE, 4, e4691. https://doi.org/10.1371/journal.pone.0004691
5. Paupy C, Kassa FK, Caron M, Nkoghé D, Leroy EM (2012) A Chikungunya Outbreak Associated with the Vector Aedes albopictus in Remote Villages of Gabon. Vector-Borne and Zoonotic Diseases, 12, 167–169. https://doi.org/10.1089/vbz.2011.0736
6. Mombouli J-V, Bitsindou P, Elion DOA, Grolla A, Feldmann H, Niama FR, Parra H-J, Munster VJ. (2013). Chikungunya Virus Infection, Brazzaville, Republic of Congo, 2011. Emerging Infectious Diseases, 19, 1542–1543. https://doi.org/10.3201/eid1909.130451
7. Venturi G, Luca MD, Fortuna C, Remoli ME, Riccardo F, Severini F, Toma L, Manso MD, Benedetti E, Caporali MG, Amendola A, Fiorentini C, Liberato CD, Giammattei R, Romi R, Pezzotti P, Rezza G, Rizzo C. (2017). Detection of a chikungunya outbreak in Central Italy, August to September 2017. Eurosurveillance, 22, 17–00646. https://doi.org/10.2807/1560-7917.es.2017.22.39.17-00646
8. de Lima Cavalcanti, T.Y.V.; Pereira, M.R.; de Paula, S.O.; Franca, R.F.d.O. (2022). A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. https://doi.org/10.3390/v14050969
9. Barbara Viginier, Lucie Cappuccio, Celine Garnier, Edwige Martin, Carine Maisse, Claire Valiente Moro, Guillaume Minard, Albin Fontaine, Sebastian Lequime, Maxime Ratinier, Frederick Arnaud, Vincent Raquin. (2023). Chikungunya intra-vector dynamics in Aedes albopictus from Lyon (France) upon exposure to a human viremia-like dose range reveals vector barrier permissiveness and supports local epidemic potential. medRxiv, ver.3, peer-reviewed and recommended by Peer Community In Infections. https://doi.org/10.1101/2022.11.06.22281997
10. Appassakij H, Khuntikij P, Kemapunmanus M, Wutthanarungsan R, Silpapojakul K (2013) Viremic profiles in CHIKV-infected cases. Transfusion, 53, 2567–2574. https://doi.org/10.1111/j.1537-2995.2012.03960.x
11. Riswari SF, Ma’roef CN, Djauhari H, Kosasih H, Perkasa A, Yudhaputri FA, Artika IM, Williams M, Ven A van der, Myint KS, Alisjahbana B, Ledermann JP, Powers AM, Jaya UA (2015) Study of viremic profile in febrile specimens of chikungunya in Bandung, Indonesia. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology, 74, 61–5. https://doi.org/10.1016/j.jcv.2015.11.017
DOI or URL of the preprint: https://doi.org/10.1101/2022.11.06.22281997
Version of the preprint: 2
PCI infections
Response to reviews
Manuscript : « Chikungunya intra-vector infection dynamics in Aedes albopictus reveals low vector barrier intensity and supports an explosive epidemic potential in mainland France »
Authors : Viginier et al.
Reviewing :
On the behalf of all authors, I thank PCI infections recommender, Dr. Sara Moutailler, for handling our manuscript as well as 2 anonymous reviewers for providing insightful comments. Detailed responses are provided below under each reviewer’s comments. For clarity, authors responses are shown in blue. Modifications of the original manuscript are reported in bold in this document and added in the revised version of the manuscript.
Reviewer 1
1. I find the title a little bit excessive when authors stated that their results support “an explosive epidemic potential in mainland France”. It has been known for a long time that chick epidemics are of the “explosive type”, as observed in La Réunion Island in 2005-2006 and also in the outbreak of 2007 in Italy, both vectorized by Ae. albopictus.
We fully agree with reviewer’s #1 comment and a revised version of the title is proposed : “Chikungunya intra-vector dynamics in Aedes albopictus from Lyon (France) upon exposure to a human viremia-like dose range reveals vector barrier’s permissiveness and supports local epidemic potential”.
Even is the study is well conducted, the results presented in this paper are not so unexpected and rely on experiments done with only one CHIKV strain, bearing the A226V substitution in the glycoprotein E1, isolated during the outbreak of La Réunion Island in 2005-2006. This strain is known to have been selected in Ae. albopictus during this outbreak and its potential of rapid and enhanced infection, dissemination and transmission in this vector have been previously well documented. Furthermore, the fact that mosquitoes could transmit as soon as 2 days post-infection has already been observed and published. Same think for dissemination rates obtained with different titers in the blood meal and RNA quantification over the time (kinetics) in whole mosquitoes, midguts and salivary glands. The plus in this study is the use of 3 different oral doses trying to span human viraemia detected in two published studies.
We do agree that the above quoted CHIKV epidemics fits with an explosive profile. However, not all the CHIKV epidemics are explosive (https://doi.org/10.3390%2Fv14050969). Importantly, while the E1-A226V mutation supports an enhanced dissemination in a laboratory population of Ae. albopictus from Galveston, USA (doi: 10.1371/journal.ppat.0030201) analysis of further VComp articles indicates that different Ae. albopictus populations present various level of vector competence for CHIKV 06.21 carrying this mutation. This is what we tried to highlight in the introduction (lines 73-75), and added at the beginning of the discussion: “Studies outlined a strong impact of mosquito and CHIKV genotype as well as temperature on Ae. albopictus potential for CHIKV transmission, including large VComp variations among worldwide Ae. albopictus populations exposed to the highly transmissible La Réunion 2006 CHIKV 06.21 isolate (Zouache et al., 2014; Mariconti et al., 2019; Gloria-Soria et al., 2020; Vega-Rúa et al., 2020). However, knowledge gaps remain regarding intra-vector virus dynamics and its impact on CHIKV epidemic potential notably regarding the virus dose.”. This underlies the importance of exposing additional populations of the Asian tiger mosquito, notably from areas at risk such as mainland France, to this viral strain.
In addition to test 3 human viraemia-like virus doses as underlined by reviewer #1, we focused on CHIKV intra-vector dynamics. The dynamic aspect is a major point of the paper. It requires the collection of individual mosquitoes at a high number of time points. As 3 oral doses were tested simultaneously, we could not logistically test more viral strains or populations within a single experiment. Intra-vector dynamics provides the advantage of allowing to determine the proportion of infectious mosquitoes over time, i.e. a quantitative estimation of vector competence with an unprecedented accuracy. In addition, the use of such data set to model CHIKV outbreaks as performed here with the agent-based model nosoi has not been performed before. This work is also a proof-of-concept study highlighting an innovative VComp measurement through dose-response intra-vector dynamics monitoring and linking VComp data with VCap using Agent Based-Model. As noticed by reviewer #1, the quantification of CHIKV in mosquito tissues has been done previously and it seemed interesting to us to confirm previously published data in an independent study. But here we demonstrate that only 12 to 25% of the mosquitoes are infectious such early after the infectious blood meal, and that is dependent on the time but not the dose. To our knowledge, our study is the first to quantify CHIKV RNA in saliva from individual mosquitoes exposed to different oral doses, showing an overall decrease of CHIKV load in the saliva over time.
For the part “Modeling chikungunya viraemia in humans” I have some questions:
2. To set up that model, authors used data from two studies, Riswari et al, 2015 and Appassaki et al, 2013. But in the result section, when presenting the estimated time course of CHIKV load in human blood (Fig 1) the model is based on the data from 5 patients, I guess described in Riswari et al, where 5 laboratory confirmed CHIK cases were identified (among a cohort of 102) and their viremia analyzed. I am not an expert in modeling, is this set of data consistent enough to draw a model? Or was I wrong in my interpretation?
Having more data would allow a better modeling and result in a smaller confidence interval. Unfortunately, these data are not available in the literature (to the best of our knowledge) as viraemia are almost never monitored over time in individual patients. But building this model is definitely possible even with the few available data, and we used to that aim a published modeling strategy (https://doi.org/10.1371/journal.pone.0083567). Overall, we found interesting to add this figure in the main text as the model fits with previous data from studies in non-human primates while it displays actual human CHIKV titers.
3. And why draw a model with those data and not simply choose different viral loads, between the upper and the lowest doses observed in patients, to infect mosquitoes? The 3 viral doses used in the infectious blood-meals are quite odd and the results difficult to compare with already published studies.
As shown in the figure, the viraemia is patient and time-dependent. Therefore, the use a global mean of all the patients at all the time points is of little interest. This is the reason why we preferred using model prediction to select a lower, medium and high dose. Notably, we knew based on previous experiments that below 3.5 log10 FFU/mL, our Ae. albopictus population was not infected by CHIKV so going below this threshold would have been pointless. That is why we started at 3.9 log10 FFU/mL as the lowest dose. The 8 log10 FFU/mL represents the maximum viraemia measured in patients of the two studies cited above, and even if a higher viraemia has been shown in the literature (up to 9 log10 FFU/mL), this was at the limit of the confidence interval of our model, while 8 log10 already result in ~100% infection and dissemination in the mosquitoes. Together, it explains the use of 8 log10 FFU/mL as the highest oral dose. Then, we selected a median dose (5.5-6 log10 FFU/mL depending on the experiment) that corresponds to a medium viraemia both before and after symptoms onset. Finally, our results indicate that the key window for mosquito infection ranges between 5 and 6.5 log10 FFU/mL, and patients with a lower or higher viraemia will likely result in very small or very high proportion of infected vectors, respectively. The range of doses used in the paper include these values. Other comparable doses have been used in previous publications, notably in the few ones that performed CHIKV dose-response experiments in Ae. albopictus (doi: 10.1089/vbz.2009.0106 , doi: 10.1371/journal.ppat.0030201 , doi: 10.1603/033.046.0228 ) as well as those using a single dose experiments (doi: 10.1128/jvi.00370-14 , doi: 10.1371/journal.pone.0059716 , doi: 10.3390/v7112917 , doi: 10.1098/rspb.2014.1078 ) allowing comparison with our study, although vector competence remains specific to mosquito and virus genotypes.
4. Also, I do not understand why two technics were performed for analyzing saliva, RNA quantification and titration on cells to detect infection viral particles. The Figure S3 give CHIKV load in each saliva even in samples without infectious CHIKV. RNA quantification is therefore not relevant, as it is already known, to calculate transmission rate, and viral load expressed as ffu/saliva would have been more informative.
Line 500-501 “when considering all CHIKV-positive salivas (including the ones with only CHIKV RNA but no infectious virus) the CHIKV RNA load of saliva… How can the authors consider positive a saliva with no infectious virus?
Initially, saliva were screened by fluorescent focus assay (FFA) for presence or absence of infectious virus. Foci were not quantified here (qualitative titration) as no serial dilutions of the samples were made, only the presence of fluorescent infected cells was recorded. Therefore, no estimation of viral titer could be made based on these analyses. However, RNA was isolated from the same saliva samples to measure CHIKV load by RT-qPCR. This allowed us to determine the viral RNA load per saliva. As RT-qPCR has a lower detection threshold compare to infectious titration, this likely explains why some saliva samples previously declared negative after FFA titration were declared positive by RT-qPCR, although with a low RNA titer. In the figure 4, we analyzed RNA load only for saliva samples that were positive after infectious titration and conclude that it is time but not dose-dependent. However, it seemed interesting to add, as supplementary information (Fig S3), that if saliva samples that were positive by RT-qPCR but negative by FFA titration were included in the analysis, the CHIKV RNA load becomes both time and dose dependent (Ptime=0.04, Pdose=0.01, Figure S3).
5. What is the reason to use a Semliki Forest virus anti-capsid antibody instead of a CHIKV antibody?
This antibody cross-react with CHIKV capsid and is used for CHIKV detection (doi: 10.1099/0022-1317-70-3-743 ). This antibody is working well on mosquitoes to detect CHIKV.
Some assertions are too definitive in the discussion:
6. Lines 466-467:
“CHIKV dissemination from the Ae. albopictus midgut depends on the interaction between time post-exposure and virus doses”.
That the conclusion of their study but CHIKV dissemination depends also on the interaction between the genotype of the virus (numerous mutations influencing dissemination have been described) and the genotype of the mosquito. The temperature is also an extremely important parameter influencing greatly the EIP and altering mosquito gene expression, bacterial microbiome and viral population diversity.
As stated elsewhere in the manuscript, we fully agree that dose and time post virus exposure are not the only factors that modulate CHIKV dissemination in Ae. albopictus. Accordingly, the discussion was reorganized to comment about time and dose-dependent VComp but also the impact of other factors (genotype, microbiota, temperature) on the intra-vector dynamics : “Genetic and environmental factors impacting intra-vector dynamics
VComp is a composite phenotype that also depends on the interaction between virus genotype, mosquito genotype and temperature (Zouache et al., 2014). From a virus perspective, some CHIKV mutations that impact VComp were already described and could be useful for epidemiological monitoring, even if data suggest that overall CHIKV swarm maintains an intermediate mutation frequency to avoid fitness loss in the mosquito (Coffey et al., 2014). Transcriptomics and quantitative genetics studies led to the identification of mosquito genetic loci that constitute interesting targets towards engineered vector control approaches, as shown for Flaviviridae (Bosio et al., 2000; Raquin et al., 2017; Aubry et al., 2020; Merkling et al., 2020;Williams et al., 2020; Dong et al., 2022). In addition, mosquitoes host a microbiota composed of bacteria, viruses, fungi and protists that have a major impact on vector’s biology (Guégan et al., 2018). Some of these micro-organisms are associated with a decrease in arbovirus transmission and constitute interesting vector control tools, like Wolbachia or Delftia tsuruhatensis bacteria blocking DENV and Plasmodium infection, respectively or insect-specific viruses modulating Ae. aegypti vector competence for DENV (Olmo et al., 2018; 2023; Huang et al., 2023). Despite an antiviral activity of Wolbachia against CHIKV in Ae. aegypti, as well a in Ae. albopictus C6/36 cell line, no blocking was detected in Ae. albopictus mosquitoes against CHIKV (Mousson et al., 2012; Raquin et al., 2015; Aliota et al., 2016). Moreover, Ae. albopictus infection by CHIKV impacts mosquito bacterial community composition while several Ae. albopictus symbionts were associated with an increase of CHIKV infection (Zouache et al., 2012; Monteiro et al., 2019). The reason for this lack of microbiota-mediated antiviral blocking against CHIKV in Ae. albopictus remains obscure but as shown for mosquito and virus genotype, Ae. albopictus microbiota composition depends on the temperature (Bellone et al., 2023). This interaction should be further studied as it could impact arbovirus transmission, as suggested by models estimating that the release of Wolbachia-infected Ae. aegypti against DENV will be less efficient upon long heatwaves due to loss of Wolbachia infection upon high temperatures (Vásquez et al., 2023). With the exception of DENV genotype (Fontaine et al., 2018), the impact of aforementioned factors was not tested on VComp dynamics and it will be interesting to determine if these factors, beyond modulating VComp at discrete time, impact the proportion of infectious mosquitoes over time.”.
7. Line 552:
“we underline the importance of testing multiple pairs of mosquito and virus genotypes to assess Vcomp in a dynamic manner, under a standardized procedure and coupled to modelling tools in order to get the most of vector competence assays.”
The abundant literature on vector competence using different epidemic viral strains and populations of mosquitoes collected on the field witness that this subject has been of concern in many studies, however this is only a part of a very complex system, with multiple parameters difficult to include in a model as mosquito immune response for instance.
As written above, we do agree with reviewer #1. In addition to the previous paragraph and in order to enlarge the discussion we propose the following modification in the discussion: “Beyond virus dose and time post virus exposure, mosquito and virus genotype, mosquito microbiota and temperature are currently identified as major VComp drivers. Therefore, locally-acquired VComp data from area at risk for arbovirus circulation are needed. This could be achieved by exposing autochthonous field-derived mosquito populations to virus strains currently circulating (or at risk of introduction) upon a range of virus dose and temperature spanning the human viremia and the mosquito season, respectively, while controlling experimentally mosquito microbiota. This is unlikely to be done within a single experiment but will require the incremental acquisition of data sets for each factor, underlying the importance of experimental procedure standardization. Interestingly, modelisation of VComp based on available data could help to target a range of the factor’s values to be tested experimentally, as exemplified with temperature (Shocket et al., 2020). These factors act together in interaction making difficult to dissociate their real impact on VComp in the field. Deciphering the complex interplay between each factor on VComp is challenging but feasible (Audsley et al., 2017) and holistic interaction studies could help to address this issue experimentally (Brinker et al., 2019). Beyond experimental conditions, our work underlines that monitoring intra-vector dynamics rather than end-point VComp is key to accurately quantify vector competence variations and better estimate VCap (Christofferson & Mores, 2011). Several studies estimated VCap through the lens of mosquito density upon environmental variations (e.g. temperature, micro-climate, land cover) but with limited ecological (mosquito survival rate, biting rate, density per host) and VComp data, notably regarding intra-vector dynamics (Murdock et al., 2017; Wimberly et al., 2020; Peña-García et al., 2023). This highlights the current need for additional VComp and VCap-related studies using field-derived material, as well as increasing efforts between vector biology and modeling fields towards an integrative VCap estimation notably regarding intra-vector arbovirus dynamics. Altogether, this will improve vector control strategies and case management by health authorities.”
8. Results described in this study are of interest, but authors used one viral strain, one mosquito population and one temperature. They should be careful not to draw too hastily general conclusions.
We thank again reviewer #1 for its helpful comments and we hope that above modifications of the manuscript will fulfill his/her expectations.
Reviewer 2
Viginier and colleagues propose and interesting manuscript around estimation of Vector competence (Vcomp) for chikungunya virus (CHIKV) transmission by Aedes albopictus from France. For this, the authors propose and original approach combining standard laboratory techniques classically used for vector competence estimations to assess CHIKV intra-vector dynamics, with a human viraemia analysis (based on data previously reported for 5 patients) and modelling through nosoi. The latter model takes into account other mosquito vector capacity (Vcap) parameters (i.e.number of bites/day) to assess the epidemic potential of CHIKV in their French Ae. albopictus strain. They overall found high permissiveness to CHIKV infection and transmission capability by the used mosquito strain, when exposed to doses that encompass human viraemia. Importantly, they showed that according to their viraemia analysis, humans are infectious to mosquitoes during more than half of their viraemia.
The manuscript fills indeed existing gaps regarding the link between human viremia window, infective doses for mosquitoes and distribution of EIP within mosquito population according to the virus dose. Authors push the experimental assay until the analysis of infectious particles and genomes in mosquito saliva which is also a major strength, so I congratulate them. Methodology is clearly described, the results overall well-presented and authors used pertinent references to discuss their findings.
We thank reviewer #2 for acknowledging the work and the strengths of our manuscript.
My questions and remarks will be the following:
Point 1: Title: I consider the titer does not perfectly fits with the paper content: “Chikungunya intra-vector infection dynamics in Aedes albopictus reveals low vector barrier intensity and supports an explosive epidemic potential in mainland France”.
The “and supports an explosive epidemic potential in mainland France” seems too speculative because even if previous studies suggest high competence of Ae. albopictus from France to transmit CHIKV, Viginier and colleagues made experiments by using a single lab colony (<10 generations) from Lyon. Given the importance of mosquito population on vector competence variations, they cannot, to my sense, extrapolate to the entire France. I suggest they reformulate the titer putting more attention on mosquito intra-barriers permissiveness for CHIKV, along with a take home message for human viraemia window that will more impactful and in agreement with their study.
This comment endorses reviewer’s #1 comment N°1 and the following title was proposed: “Chikungunya intra-vector dynamics in Aedes albopictus from Lyon (France) upon exposure to a human viremia-like dose range reveals vector barrier’s permissiveness and supports local epidemic potential”.
Point 3: My other major comment concerns the discussion. While overall well written, authors spend time comparing ZIKV and CHIKV replication in mosquitoes, which I consider not so relevant (viruses from different families with different replication strategies in the cell) regarding the aims of the study. I suggest authors discuss more on factors/methodologies that could finely improve the final Vcap-Vcomp estimations in the models, or the gaps on data collection to add those criteria. More extensive discussion on competence variations across populations could also be appreciated.
Our goal was to put CHIKV/ZIKV differences in replication strategy in perspective with VComp, including VComp dynamics as ZIKV is the only mosquito-borne virus for which intra-vector dynamics was monitored in a dose-dependent manner in Ae. albopictus, although from a different area in France (doi: 10.1371/journal.ppat.1009068 ). We found that both viruses present a very close OID50% but very different intra-vector dynamics that could be due linked with mosquito population and/or virus replication strategy.
As hinted by reviewer #2, VComp and VCap are different read-out and cannot be addressed the same way. For VComp, which is the main topic of the paper, the discussion on the factors that drives VComp (beyond time and dose) and how to address them from a methodological perspective could be improved. As pointed out by reviewer #1, mosquito and virus genotypes, mosquito microbiota and temperature are the other currently identified main drivers of VComp. This prompts the need for additional studies on these factors, taking also into account their potential interactions. VComp dynamics will allow a better estimation of VCap, that would also benefit from local mosquito ecological data acquisition, provided that modelling approaches goes toward more integrative models. Accordingly and as mentioned above, the following paragraph was added to the discussion: “Beyond virus dose and time post virus exposure, mosquito and virus genotype, mosquito microbiota and temperature are currently identified as major VComp drivers. Therefore, locally-acquired VComp data from area at risk for arbovirus circulation are needed. This could be achieved by exposing autochthonous field-derived mosquito populations to virus strains currently circulating (or at risk of introduction) upon a range of virus dose and temperature spanning the human viremia and the mosquito season, respectively, while controlling experimentally mosquito microbiota. This is unlikely to be done within a single experiment but will require the incremental acquisition of data sets for each factor, underlying the importance of experimental procedure standardization. Interestingly, modelisation of VComp based on available data could help to target a range of the factor’s values to be tested experimentally, as exemplified with temperature (Shocket et al., 2020). These factors act together in interaction making difficult to dissociate their real impact on VComp in the field. Disentangling the complex interplay between each factor on VComp is challenging but feasible (Audsley et al., 2017) and holistic interaction studies could help to address this issue experimentally (Brinker et al., 2019). Beyond experimental conditions, our work underlines that monitoring intra-vector dynamics rather than end-point VComp is key to accurately quantify vector competence variations and better estimate VCap (Christofferson & Mores, 2011). Several studies estimated VCap through the lens of mosquito density upon environmental variations (e.g. temperature, micro-climate, land cover) but with limited ecological (mosquito survival rate, biting rate, density per host) and VComp data, notably regarding intra-vector dynamics (Murdock et al., 2017; Wimberly et al., 2020; Peña-García et al., 2023). This highlights the current need for additional VComp and VCap-related studies using field-derived material, as well as increasing efforts between vector biology and modeling fields towards an integrative VCap estimation notably regarding intra-vector arbovirus dynamics. Altogether, this will improve vector control strategies and case management by health authorities.”.
Regarding VComp variations among mosquito populations, it is hard to disentangle the impact of the mosquito genotype from the one of the virus as this is the interaction between both that drives VComp, in addition to other factors mentioned above. Following on reviewer’s #1 comment, we emphasized that even for La Réunion 2006 CHIKV 06.21 isolate (carrying an adaptative E1 A226V mutation that increases its transmission by Ae. albopictus), strong VComp differences can be seen among worldwide Ae. albopictus populations orally exposed to that specific isolate. These sentences were added to the discussion: “Studies outlined a strong impact of mosquito and CHIKV genotype as well as temperature on Ae. albopictus potential for CHIKV transmission, including large VComp variations among worldwide Ae. albopictus populations exposed to the highly transmissible La Réunion 2006 CHIKV 06.21 isolate (Zouache et al., 2014; Mariconti et al., 2019; Gloria-Soria et al., 2020; Vega-Rúa et al., 2020). However, knowledge gaps remain regarding intra-vector virus dynamics and its impact on CHIKV epidemic potential notably regarding the virus dose.”.
Point 4: Recent studies showed that arboviruses dissemination in mosquitoes increase with gonotrophic cycles. This is pertinent for design methodology and also for modelling Vcomp and vectorial capacity. Authors should discuss on how this may impact their estimations.
We found a reference (https://doi.org/10.3390%2Fv15051043) on the importance of the number of gonotrophic cycles on CHIKV dissemination. Regarding the impact of gonotrophic cycles on VComp, the article shows a 12% increase (87 to 99%) in dissemination for CHIKV in Ae. aegypti, while in comparison, Armstrong et al. (2019) show a 27% increase (29 to 56%) in CHIKV dissemination between mosquitoes fed with one or two blood meals consecutively, that results in 1.5 days shorter EIP for the second group. Altogether, it seems indeed important to mention both studies in the discussion although more data are needed to further discuss their integration in VCap estimation. Therefore, the discussion was modified as follows : “This also requires other improvements of modelling strategies to account for mosquito and human populations structure, sanitary measures as well as Ae. albopictus tendency to take several consecutive blood meals, as virus dissemination increases with gonotrophic cycles and that successive bloodmeals are associated with a shortened EIP (Delatte et al., 2010; Armstrong et al., 2019; Fikrig & Harrington, 2021; Mulatier et al., 2023).”.
Point 5: Their vector competence estimation could also be influenced by mosquito maintenance in controlled facilities. Authors could comment on that.
It is likely that colony maintenance impacts the genetic structure of the population by selecting only the females that fed then laid viable eggs. Here, mosquitoes were reared in mass (thousands of individuals per cage) using mice feeding. Thanks to a high feeding rate, a certain level of genetic diversity could be maintained thereby limiting the genetic bottleneck that arises from colonization. The material and methods section was modified accordingly: “Mass rearing of the population under standard laboratory conditions (28°C, 80% relative humidity, 16:8 hours light:dark cycles) using mice feeding (Mus musculus) allowed to maintain genetic diversity, in accordance with the Institutional Animal Care and Use Committee from Lyon1 University and the French Ministry for Higher Education and Research (Apafis #31807-2021052715018315).”.
Point 6: A question: The number of analyzed mosquitoes (figure 2) is different for the doses used, with lower number with the highest dose. Why such disparity? Higher mortality was observed at 8.63 dose? If so, this could be mentioned and discussed if authors could quantify that effect.
No additional mortality was detected according to oral dose, the mortality rate being very low overall (<5%). We knew from pilot experiments that highest dose will lead to almost 100% infection and dissemination while at lowest dose, more individuals will be needed to get an accurate estimation of IR and DR. Accordingly, and as all the doses were tested concomitantly within each experiment, more feeding boxes were prepared for the lowest dose explaining the differences in sample size. This was added in the result section: “The mortality rate was very low regardless of time or oral dose, remaining below 5%.”.
Minor remarks:
Line 155: please state the exact generation
This was done, line 169.
Line 312: “separately exposed”
Corrected.
There are minor grammar corrections to be done on lines 416, 421, 506
Corrected.
 , posted 23 Jul 2023, validated 25 Jul 2023
, posted 23 Jul 2023, validated 25 Jul 2023Dear authors
Please follow the recommandations of the 2 reviewers to improve your preprint.
Regards
Sara Moutailler
I find the title a little bit excessive when authors stated that their results support “an explosive epidemic potential in mainland France”.
It has been known for a long time that chick epidemics are of the “explosive type”, as observed in La Réunion Island in 2005-2006 and also in the outbreak of 2007 in Italy, both vectorized by Ae. albopictus.
Even is the study is well conducted, the results presented in this paper are not so unexpected and rely on experiments done with only one CHIKV strain, bearing the A226V substitution in the glycoprotein E1, isolated during the outbreak of La Réunion Island in 2005-2006. This strain is known to have been selected in Ae. albopictus during this outbreak and its potential of rapid and enhanced infection, dissemination and transmission in this vector have been previously well documented.
Furthermore, the fact that mosquitoes could transmit as soon as 2 days post-infection has already been observed and published. Same think for dissemination rates obtained with different titers in the blood meal and RNA quantification over the time (kinetics) in whole mosquitoes, midguts and salivary glands. The plus in this study is the use of 3 different oral doses trying to span human viraemia detected in two published studies.
For the part “Modeling chikungunya viraemia in humans” I have some questions:
To set up that model, authors used data from two studies, Riswari et al, 2015 and Appassaki et al, 2013. But in the result section, when presenting the estimated time course of CHIKV load in human blood (Fig 1) the model is based on the data from 5 patients, I guess described in Riswari et al, where 5 laboratory confirmed CHIK cases were identified (among a cohort of 102) and their viremia analyzed. I am not an expert in modeling, is this set of data consistent enough to draw a model? Or was I wrong in my interpretation?
And why draw a model with those data and not simply choose different viral loads, between the upper and the lowest doses observed in patients, to infect mosquitoes? The 3 viral doses used in the infectious blood-meals are quite odd and the results difficult to compare with already published studies.
Also, I do not understand why two technics were performed for analyzing saliva, RNA quantification and titration on cells to detect infection viral particles. The Figure S3 give CHIKV load in each saliva even in samples without infectious CHIKV. RNA quantification is therefore not relevant, as it is already known, to calculate transmission rate, and viral load expressed as ffu/saliva would have been more informative.
Line 500-501 “when considering all CHIKV-positive salivas (including the ones with only CHIKV RNA but no infectious virus) the CHIKV RNA load of saliva….
How can the authors consider positive a saliva with no infectious virus?
What is the reason to use a Semliki Forest virus anti-capsid antibody instead of a CHIKV antibody?
Some assertions are too definitive in the discussion:
Lines 466-467:
“CHIKV dissemination from the Ae. albopictus midgut depends on the interaction between time post-exposure and virus doses”.
That the conclusion of their study but CHIKV dissemination depends also on the interaction between the genotype of the virus (numerous mutations influencing dissemination have been described) and the genotype of the mosquito. The temperature is also an extremely important parameter influencing greatly the EIP and altering mosquito gene expression, bacterial microbiome and viral population diversity.
Line 552
“we underline the importance of testing multiple pairs of mosquito and virus genotypes to assess Vcomp in a dynamic manner, under a standardized procedure and coupled to modelling tools in order to get the most of vector competence assays.”
The abundant literature on vector competence using different epidemic viral strains and populations of mosquitoes collected on the field witness that this subject has been of concern in many studies, however this is only a part of a very complex system, with multiple parameters difficult to include in a model as mosquito immune response for instance.
Results described in this study are of interest, but authors used one viral strain, one mosquito population and one temperature. They should be careful not to draw too hastily general conclusions.
Viginier and colleagues propose and interesting manuscript around estimation of Vector competence (Vcomp) for chikungunya virus (CHIKV) transmission by Aedes albopictus from France. For this, the authors propose and original approach combining standard laboratory techniques classically used for vector competence estimations to assess CHIKV intra-vector dynamics, with a human viraemia analysis (based on data previously reported for 5 patients) and modelling through nosoi. The latter model takes into account other mosquito vector capacity (Vcap) parameters (i.e. number of bites/day) to assess the epidemic potential of CHIKV in their French Ae. albopictus strain. They overall found high permissiveness to CHIKV infection and transmission capability by the used mosquito strain, when exposed to doses that encompass human viraemia. Importantly, they showed that according to their viraemia analysis, humans are infectious to mosquitoes during more than half of their viraemia.
The manuscript fills indeed existing gaps regarding the link between human viremia window, infective doses for mosquitoes and distribution of EIP within mosquito population according to the virus dose. Authors push the experimental assay until the analysis of infectious particles and genomes in mosquito saliva which is also a major strength, so I congratulate them. Methodology is clearly described, the results overall well-presented and authors used pertinent references to discuss their findings.
My questions and remarks will be the following:
Point 1: Title: I consider the titer does not perfectly fits with the paper content: “Chikungunya intra-vector infection dynamics in Aedes albopictus reveals low vector barrier intensity and supports an explosive epidemic potential in mainland France”
The “and supports an explosive epidemic potential in mainland France” seems to speculative because even if previous studies suggest high competence of Ae. albopictus from France to transmit CHIKV, Viginier and colleagues made experiments by using a single lab colony (<10 generations) from Lyon. Given the importance of mosquito population on vector competence variations, they cannot, to my sense, extrapolate to the entire France.
I suggest they reformulate the titer putting more attention on mosquito intra-barriers permissiveness for CHIKV, along with a take home message for human viraemia window that will more impactful and in
agreement with their study.
Point 3: My other major comment concerns the discussion. While overall well written, authors spend time comparing ZIKV and CHIKV replication in mosquitoes, which I consider not so relevant (viruses from different families with different replication strategies in the cell) regarding the aims of the study. I suggest authors discuss more on factors/methodologies that could finely improve the final Vcap-Vcomp estimations in the models, or the gaps on data collection to add those criteria. More extensive discussion on competence variations across populations could also be appreciated.
Point 4: Recent studies showed that arboviruses dissemination in mosquitoes increase with gonotrophic cycles. This is pertinent for design methodology and also for modelling Vcomp and vectorial capacity. Authors should discuss on how this may impact their estimations.
Point 5: Their vector competence estimation could also be influenced by mosquito maintenance in controlled facilities. Authors could comment on that.
Point 6: A question: The number of analyzed mosquitoes (figure 2) is different for the doses used, with lower number with the highest dose. Why such disparity? Higher mortality was observed at 8.63 dose? If so, this could be mentioned and discussed if authors could quantify that effect.
Minor remarks:
Line 155: please state the exact generation
Line 312: “separately exposed”
There are minor grammar corrections to be done on lines 416, 421, 506